What is "highly enriched" uranium?
And how highly enriched does it have to be?

Natural Uranium – contains a 238U concentration of 99.27 percent, 235U concentration of 0.711 percent and very little 234U.


So U-235 is "fissile" while U-238 is not. But U-238 absorbs neutrons and decays into Plutonium-239 which is also fissile.
Plutonium-239 is 29,000 times more radioactive than Uranium-235 and its fission reactions are prone to accidental prompt criticality and generally much more difficult to control.
These are the limitations and realities of designing and operating safe and effective nuclear power reactors as opposed to nuclear weapons.
Rods, pebbles or other piles of low enriched uranium are only allowed to react partially to a slight extent in a power plant, and no complete burn or utilization of the energy theoretically available from the fission of uranium and plutonium is ever achieved because the partially used fuel has to be pulled out and sent to long term storage when too much U-235 has been consumed and too much U-238 has been converted into Pu-239 by absorbing neutrons and emitting beta particles.

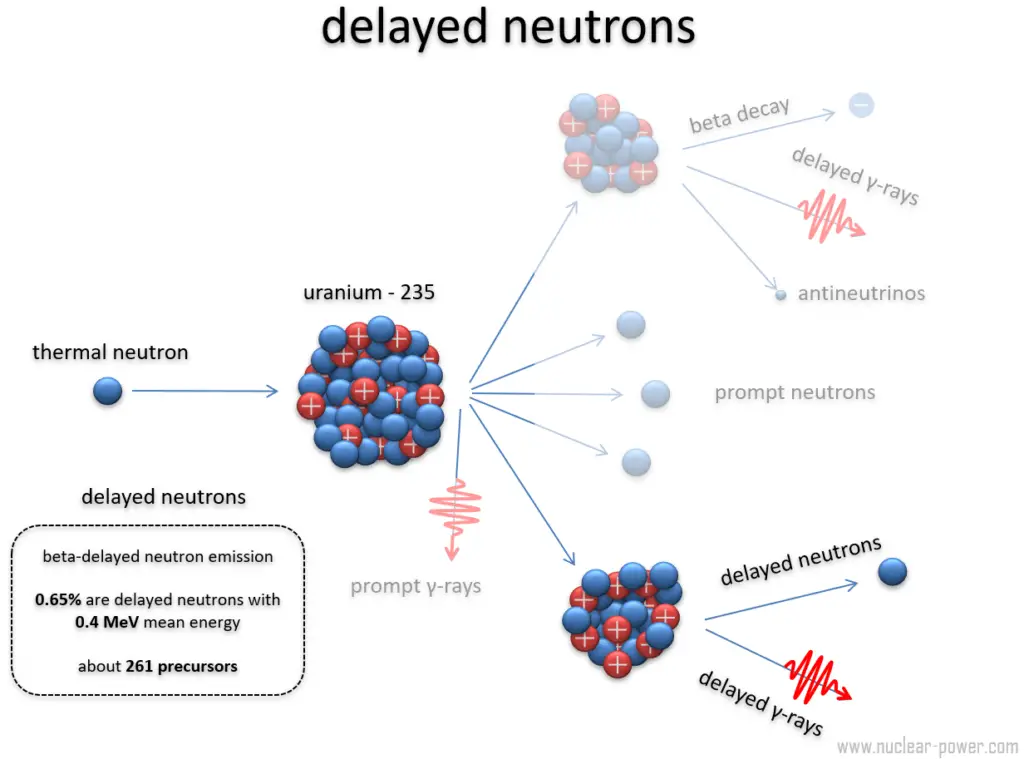
The "moderation" of nuclear fission in a power plant — that is, the ability to produce power without causing a nuclear explosion — depends critically on the availability of delayed neutrons.
The reaction cannot be allowed to go critical with prompt neutrons alone, because that would instantly cause a nuclear explosion. However the prompt and delayed neutrons together much be enough to achieve and maintain criticality for power production.
Almost all the neutrons emitted in the fission of Plutonium-239 are prompt, and it is somewhat more difficult to maintain a controlled reaction without causing a nuclear explosion when too much of this isotope has accumulated as "waste."
A plutonium power plant could easily be built and operated to effectively use waste Plutonium-239 and depleted Uranium-238 as primary fuels by incorporating some neutron-moderating elements such as aluminum and possibly boron.